Fair Scientific Consulting
Contact us: 801-644-8800
Email: info@fairscientificconsulting.
Who We Are
Fair Scientific Consulting
Our team of skilled lab techs, quality managers, certified quality auditors, and laboratory directors have cumulative experience in participating or leading almost 100 laboratory/facility audits and inspections from CLIA, CAP, COLA, TJC, ISO, FDA-GLP, and OSHA.
We work with laboratories being built from scratch, eliminating any worry of meeting regulatory requirements.
We help regulatory deficient laboratories fix and clear up flaws, becoming compliant as soon as possible.
We work on-site validating new assays, and training techs to take patient samples. Validations have been completed for FDA-approved assays, EUA assays, and non-FDA approved Laboratory Developed Tests (LDTs).
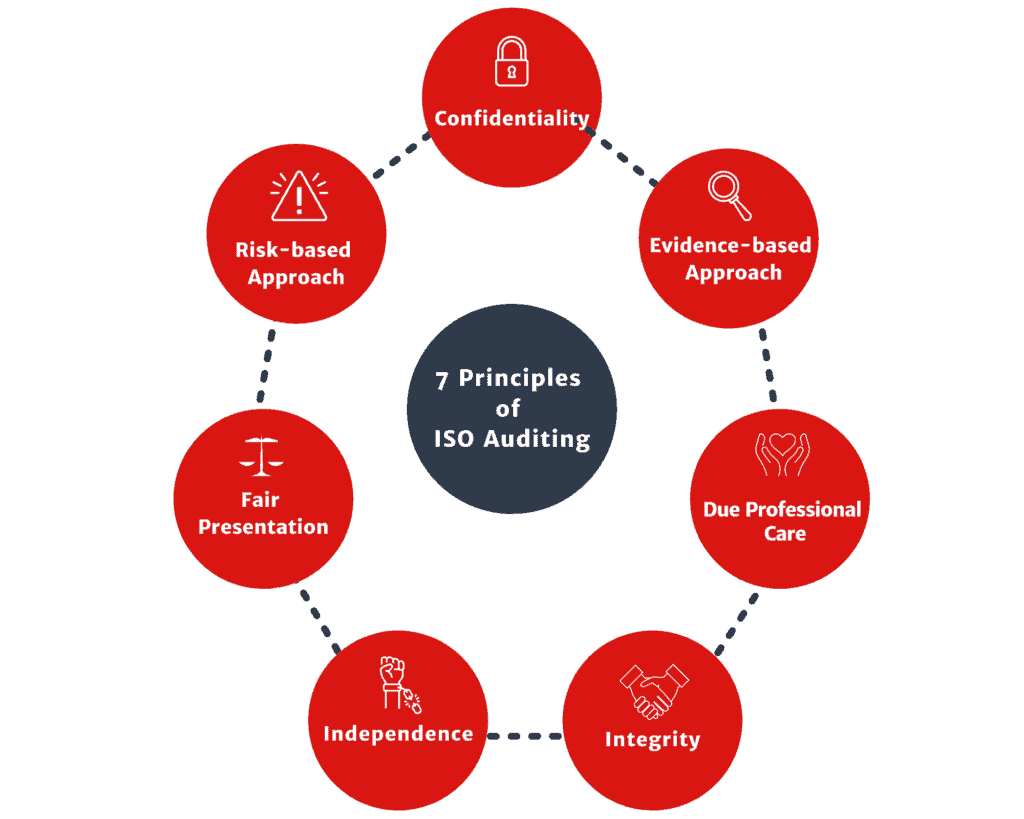
Our company works within the 7 principles of ISO auditing and are proud to serve our clients using these guidelines.
Services
Our Lab Services
Validations
The process of demonstrating that the procedure being performed can be done under the specific environmental conditions and requirements of the laboratory.
FSC will provide validations services under GLP and CLIA/COLA/CAP regulations to support clinical diagnostic testing of your assay with your specific instrumentation.
Laboratory Developed Tests
LDT’s for your lab are assays that you have designed or another facility has designed that are not FDA-approved assays. These assays are intended for clinical use and need to be validated to be used within the laboratory.
The lab needs to establish acceptable performance by analytical and clinical validations.
Lab Design and Workflow
Fair Scientific is skilled in the process of design, build-out, and set up, helping new laboratories be efficient, in space and budget, for workflow processes.

Technical Consultant
and Lab Director
Ongoing support with structured site visits to perform reviews and any competency evaluations needed to maintain the integrity of the lab.
Personnel Training
and Management
Provide ongoing support for
- Hiring skilled laboratory staff
- Performing competency training
- Training for new assays and instrumentation
- Training in laboratory safety
- Good Document Practice
- QA/QC
And any other training needed within your laboratory.
Regulatory Affairs and Quality Assessment
Professional assistance with your regulatory affairs, document control, quality assurance plans, and technical review required by your regulatory agency that needs to be maintained in every lab.

Expertise in
ASSAY Validation
Women's Health Panel
Blood Chemistry
Pharmacogenomics PGx
Toxicology and Drugs of Abuse Testing
Inherited Disease
Cancer Genetics
CGx/Hereditary Cancer
Complete Wellness Panel
Infectious Disease
Urinary Tract Infection (UTI) Panel with Antibiotic Resistance
COVID-19 + Respiratory Pathogen Panel (RPP)
Antibody Susceptibility Testing (AST)
Comprehensive Wound / Infection Panel
Gastrointestinal (GI) Panel
Sexually Transmitted Infection Panel
Nail Fungus Panel

Expertise
Technical specialties
- Clinical Validations for New Assays
- Laboratory Developed Test Validations
- Instrument Evaluation and Performance Verification
- Personnel Competencies and Training
- QA/QC, IQCP, and Quality Management
- Technical Supervisor and/or Technical Consultant for a variety of specialities.



Consultation
Laboratory Management and Regulatory Agency Certification for CLIA, COLA, CAP, TJC, and ISO.
- Regulatory Agency Application Processing
- Customized Laboratory Workflow and Efficiency Design with ability to use Six Sigma Black Belt specialists.
- Laboratory Proficiency Testing Setup and assistance with events.
- CAPA / RCA and Risk Management
- Inspection Preparation with Mock Audits
- Document Control and Personnel Competency Assessment Control
- LIS and EMR Establishment and Setup

Auditing
LABORATORY MOCK AUDITING
- Audits include the whole laboratory path of workflow with the outcome of providing a thorough report of any problems detected throughout the process
- Assessment of Quality System Essentials
- Evaluation of Organizational Workflow, Process Control, and Process Improvement
- Review of Personnel Documentation, Qualifications, and Competency
- Assessment of Documents and Records
- Examination of Facilities and Safety Records


Get Accredited









Fair Scientific Consulting, LLC 2020, All rights reserved
